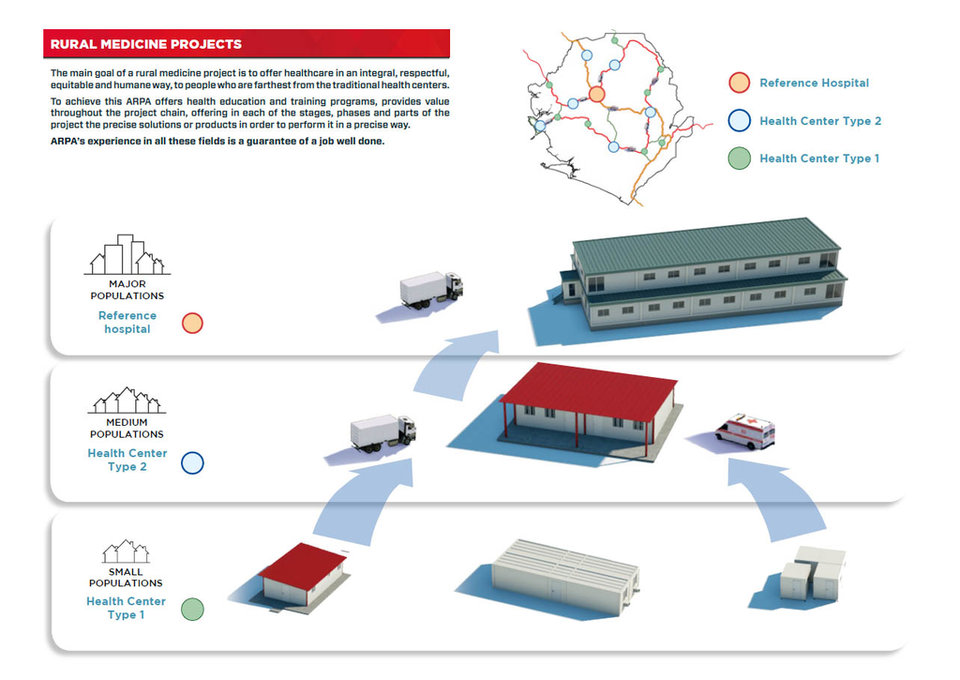
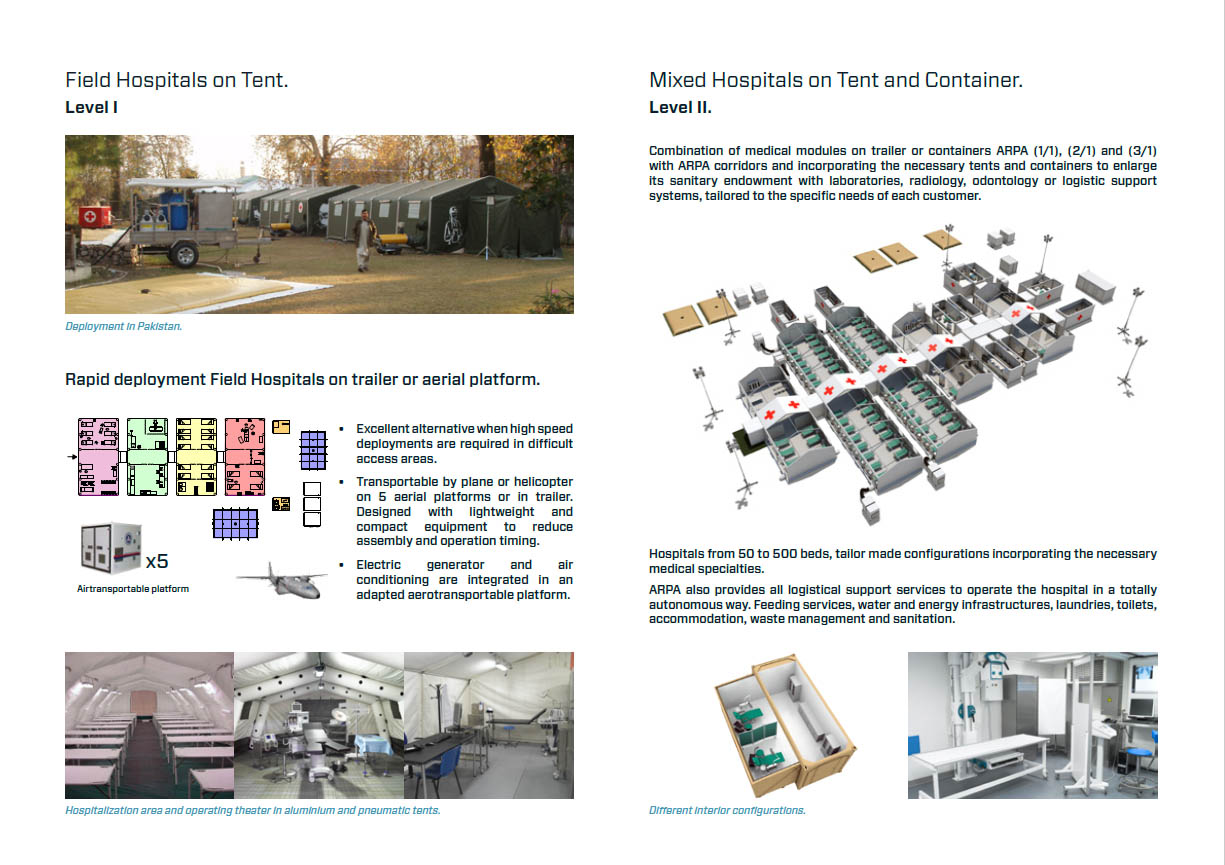
I
njuries and conditions leading to lower limb disorders, including spinal cord injuries and cerebral embolisms, can feel like life-ruining events to patients. Treatments, if any can be prescribed, can be lengthy and uncomfortable, often leading to extreme frustration and despair. In the US alone, an estimated 10,000 to 12,000 spinal cord injuries occur every year, with around 200,000 Americans currently living with related conditions.
The Hybrid Assistive Limb (HAL) is a powered exoskeleton suit developed by Japanese robotics technology company Cyberdyne that has been used in Japanese medical institutions since 2008 for the treatment of lower limb disorders. By 2012 more than 300 HAL suits were in use across Japan, by 130 different medical facilities. Now the technology has made its way over to the US, receiving Food and Drug Administration (FDA) clearance in 2017.
Brooks Rehabilitation, post-acute physical rehabilitation specialists based in Florida, announced a partnership Cyberdyne in March 2018 – since then it has become the first US-based provider of HAL treatments through the new Brooks Cybernic Treatment Center in Jacksonville, Florida.