From R&D to Final Release
Pave your way to market by controlling quality with smart and robust Sartorius solutions. From simple needles, bandages or surgical instruments to complex drug-eluting stents or pacemakers – medical device manufacturing comes with stringent quality, safety and efficacy standards.
Let Sartorius support you in maintaining reliable and risk-mitigated processes, delivering high-quality products and ensuring regulatory compliance.
Best-fit solutions assist you through the entire development and production process to fulfill biocompatibility, cleanliness, safety and packaging standards.Benefit from precision weighing solutions, ultra-pure water systems and our expertise in filtration, topped with Data Analytics software for straightforward and reliable QC.
With over 150 years of trusted service and proven innovation, quality and reliability, Sartorius can help you bring your life-enhancing medical devices safely and efficiently to market.
Learn more about our Medical Device Solutions
Implants
Surgical Instruments

Diagnostic Kits

Contract Manufacturing & Integration

Wearable & Disposables

Drug Delivery Systems
Medical Device Quality control Made Easy
Medical devices - General Process Steps
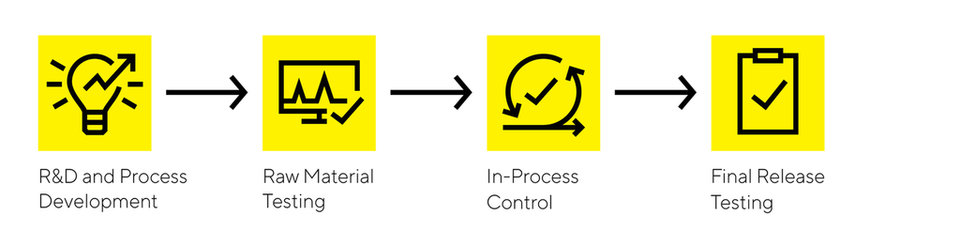

R&D and Process Development
Quality control starts in the design phase and continues through the whole product life cycle. It is vital activity for successful commercialization, assuring the consistent and compliant manufacturing of life enhancing products.
Discover R&D and Process Development solutions for your application:
Density Check of Solid Material & Liquids
Nanoparticle Concentration
Analytical Characterization - Biocompatibility
Cytotoxicity - Biocompatibility
Data Analytics

Raw Material Testing
The quality of the final product hinges on the quality of the raw materials. To prevent device defects and impairment of patient safety, your raw materials must be stable and safe for use.
Discover the Raw Material Testing solutions for your application:
Density Check of Solid Material & Liquids
Moisture Determination of Raw Material
Particulate Matter Analysis
Analytical Characterization - Biocompatibility
Microbial Testing: Water & Bioburden Testing


In-Process Control
In-process quality control tests are performed at regular intervals to establish that product quality and specifications are met at all stages of production.
Discover the In-Process Control solution for your application:
Check Coatings – Application of Correct Amounts
Moisture Determination
Nanoparticle Filtration of Liquids - Concentration – Detection
Particulate Matter Analysis
Continuous Microbial Air Monitoring
Rinsing & Sterile Preparation
Data Analytics
Counting with Reference Weight

Final Release Testing
Every lot requires tests to ensure each implant is free of contaminants. Error-free assembly, conforming to controlled tolerances, is necessary to avoid malfunction.
Discover the Final Release Testing solutions for your application:
Check Coatings Application
Error-Proof Packaging
Density Check of Solid Material & Liquids
Moisture Determination of Packaging Material
Microbial Testing: Bioburden & Sterility
Analytical Characterization - Biocompatibility

Contract Manufacturing &
Integration, a Partnership to Rely on
Simple. Flexible. Reliable. For Best Performance and Design.
Learn more about our Medical Device Solutions
Customized Solutions, Taylor Made For
Your Individual Process