Insight
Is software a blind spot for the medical device industry?
Software is driving incredible advancements in medical technology and opening up exciting opportunities for decentralised healthcare. But, at the same time software is also the leading cause of device recalls and failures prompting calls for a shift in focus. Has there been too much focus on hardware? Chris Lo reports.

A
s the provision of healthcare continues its transition towards preventative care, software and complex algorithms have formed the basis for a revolution in health data collection and analytics. At the patient level, that means new opportunities are opening up in remote patient monitoring (RPM), wearable devices and advanced diagnostics, giving users access to high-quality medical technologies that can be accessed outside the hospital, or even at home.
Software is obviously nothing new in the medtech world. Jeff LeBlanc, director of user experience at user interface (UI) design firm Boston UX, remembers his earliest job in the medical device field three decades ago, working on cardiac monitoring devices for Hewlett Packard.
“I was doing what I considered software even then,” he says. “I started by drawing medical waveforms on a screen and trying to duplicate in software what has historically been done with analogue devices.”
The difference today is the complexity of the modern data environment and the central role that software is playing in decentralising medical technology. As connectivity becomes king for medical devices, software has moved from the industry’s back seat to take the wheel. And with the pace of innovation in software, disruptive ideas are being presented and implemented faster than ever before.
“We have so much flexibility with what we can do in software,” LeBlanc says. “We can change quickly, we can innovate quickly, which lets us get better solutions out to healthcare providers faster and faster.”
That’s the pitch, anyway.

Director of user experience at Boston UX, Jeff LeBlanc.
Image courtesy of ICS
Software: a potential blind spot for medical device firms
While the promise of modern medical software is real and incredible advances have been achieved, the complexity of today’s connected systems makes software a sticking point in the industry.
Software failure is a leading cause of medical device recalls; according to research by Stericycle Expert Solutions, which publishes a quarterly product recall index, Q3 2018 was the tenth consecutive quarter in which software issues were the leading cause of medical device recalls at 22% of the total. The statistics reflect, Stericycle said, “the increasing technological sophistication and complexities of modern medical devices as they become part of the expanding internet of things [IoT]”.
How well an application is put together can determine how effective a clinician is in delivering care.
Even the most established device firms are susceptible to software issues. In March, the US Food and Drug Administration (FDA) issued a Class I recall – the most serious category of recall for devices that pose a risk of serious injury or death – of a range of Medtronic dual-chamber implantable pulse generators, pacemakers that deliver stimulation to patients with bradycardia. The cause for the recall of more than 13,000 devices was a software error that can “result in a lack of pacing”, and the FDA noted that “patients and physicians cannot predict whether and when this software error might occur”.
With patient safety at stake and software issues lying at the heart of many device failures, what’s going wrong in the industry? Part of the explanation could be that medical device companies have traditionally been focused on physical hardware, leaving software as something of a blind spot.
“It’s different disciplines,” says Sam Liu, marketing director at California-based patient monitoring company VivaLNK. “If you’re a company that’s primarily focused on hardware, for many reasons you’re not necessarily very good at software, and vice versa. In software you get into more analytics and algorithms, and sometimes it’s the user interface – how well an application is put together can determine how effective a clinician is in delivering care. Those things often become a second thought, not the primary focus when you’re a device manufacturer. In order for the medical industry to really grow, there needs to be some separation of the hardware devices from the innovative software that can go inside them.”

Sam Liu, marketing director at VivaLNK. Image courtesy of VivaLNK
Medical software: a focus on usability
Designing and engineering software that responds as expected in a safety-critical environment is tough enough, but designing UIs that minimise user error and make sense to medical professionals (or even patients) who don’t have software engineering degrees, adds another layer of complexity. And developing a user experience that isn’t confusing is just as critical to patient safety as the reliability of the software itself.
“There are stories years ago about patients who were connected to these infusion pumps, and were delivered lethal doses of medication because the software allowed the user to do something that they shouldn’t,” says LeBlanc. “They push that button too many times, and it has lethal consequences. It’s horrible. But we can design around that and prevent that type of thing from happening.”
So what are the UI design principles that can minimise the risk of user error?
What’s intuitive to an engineer isn’t necessarily intuitive to a non-engineer.
“Making it intuitive to the end user,” LeBlanc says. “Knowing the types of equipment they’re familiar with, and designing something so they can transfer their knowledge from one device to another. Knowing their environment and designing around that, trying to make our interfaces adapt to what these high-stress environments are. What’s intuitive to an engineer isn’t necessarily intuitive to a non-engineer. If we look at our users, at least half of them have an iPhone these days and they know how to use it. They understand those paradigms, so we leverage that.”
For medical staff, and especially for patients themselves as remote devices start crossing the threshold into the home, that might mean that device manufacturers and their software partners should be taking more cues from consumer tech; the Apples and Googles of the world.
“Look at companies that put in the time and effort to create these good experiences and emulate them,” LeBlanc advises. “That doesn’t necessarily mean just make it look like an iPhone – although that might end up being some of the result – but understand the design process that went on for those companies to get there, and then go through the same process yourself.”
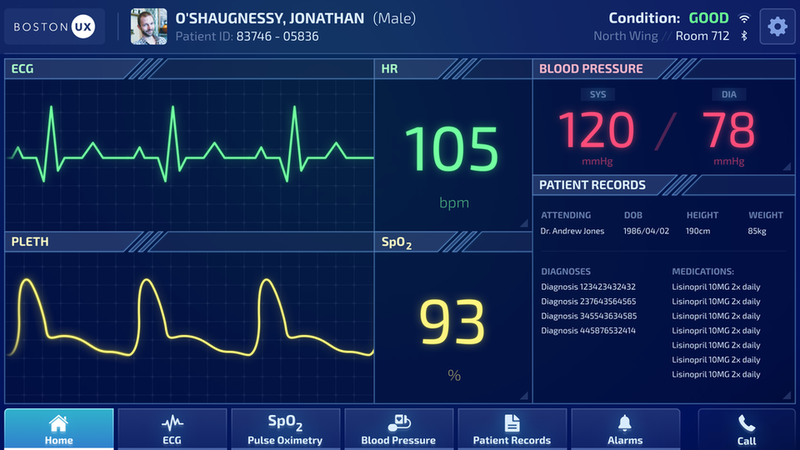
Boston UX Device. Image courtesy of Boston UX
From solutions to platforms
The immense challenge of designing hardware and software that acts in harmony in a single system could be a tripping-up point for an industry that is looking to push connected devices as the key to a new eco-system of remote monitoring and preventative care devices.
VivaLNK is looking to become part of the solution with the recent launch of its IoT-enabled medical wearable Sensor Platform. The hardware/software system, which is intended for continuous patient monitoring, incorporates an array of sensor patches as well as a software system to handle the collection and management of biometric data from the patient.
Our approach is to look at the relevant human vitals and biometrics across the board and consolidate that into a single platform.
The whole system is being offered as a platform that software developers can use as the basis for their own analytical applications, which Liu says is currently a rare offer but is a growing trend in the sector – he points to consumer heart rate monitor firm Polar, which has also adopted the platform approach with the launch of its own software development kit.
“Our approach is to look at the relevant human vitals and biometrics across the board and consolidate that into a single platform so a software developer only has to deal with one programming protocol. That will make their job far easier.”
“The delivery of data from the sensors on the patient to the application is also handled by the platform, so the application won’t have to worry about connection losses and recovery and caching and synchronisation,” he says. “We have one partner that’s making a chemo treatment application based on multiple vitals. So now all they have to worry about is, ‘Okay, I want vitals A, B and C – I’m not going to worry about how I get it or whether I get it. I can just focus on my algorithms and application to process those vitals for analysis.’”
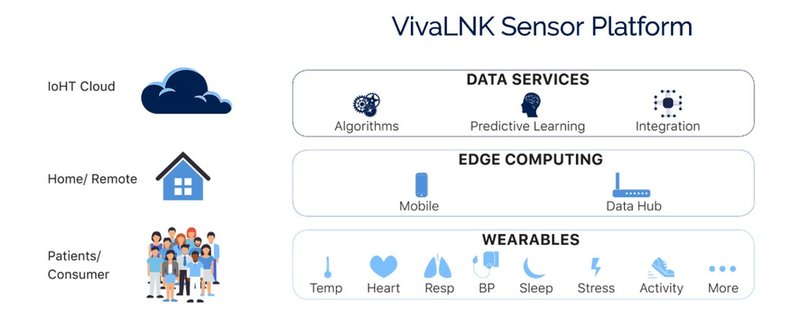
VivaLNK sensor platform - Image courtesy of VivaLNK
The future for decentralised healthcare
Liu argues that offering a platform that allows specialist developers to concentrate on their own innovations rather than the intricacies of hardware and device regulation will help accelerate the transition towards healthcare decentralisation – a process that will almost certainly be supported by mainstream tech companies and which could leave the large device companies behind if they’re not moving with it.
“The macro-trend in healthcare is moving from very centralised, monolithic environments to a more distributed environment. And when you do that, the traditional players that are used to either a certain cost structure, or certain domain expertise in very large systems, they’re not going to be very efficient in a world where it’s more distributed at a lower cost. That trend is forcing a different kind of innovation that the big guys usually have a hard time keeping up with.”
There are thousands of patients at any given moment whose lives may be dependent on their software.
Still, safety must always come first. The transformative potential of innovative medical software is clear, but this disruptive process must be based on incredibly high standards of reliability, cybersecurity and intuitive design. Most in the industry need little reminding of that fact, as there are thousands of patients at any given moment whose lives may be dependent on their software.
“Safety in the medical industry is very personal to me because I’ve seen family members hooked up, and I’ve always thought, ‘Boy, I hope we got this right,’” says LeBlanc. “Having designers get out into the field to see how real this is – it’s not an academic problem, it’s a personal problem that impacts all of us. If we’re working in the medical industry, somebody that we know will probably be impacted by one of our devices, so that should be pretty high motivation for people to get it right.”