Silbione™ Biomedical: an extended range of implantable-grade Silicones
Silbione™ Biomedical Grade silicone materials are designed and manufactured to meet the strict regulatory, quality, and purity requirements of the long-term implant (>29 days in the body) and drug delivery markets.
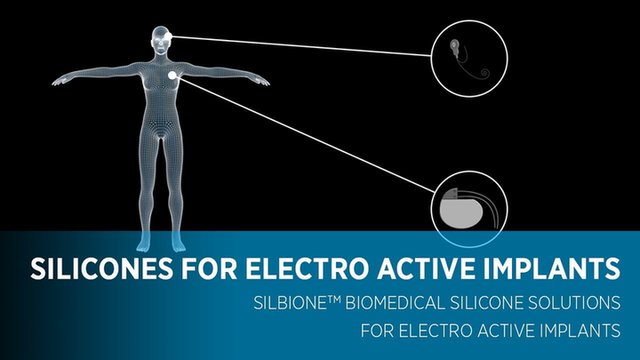
Silicones are material of choice for certain pieces that can be implanted into the body such as Nasal implants for reconstructive surgery, Cochlear implants, gastric bands or heart valve and pacemakers Click on the different parts of the body below and discover numbers of silicone elastomers used as implantable medical grades:
Click on circles for more infomation

Our latest innovation is a tin-free, quick cure implantable-grade adhesive which can be used in the assembly of implanted devices such as pacemakers.
The Elkem Silicones Silbione™ Biomedical ADH1 M200 implant grade adhesive is a single component silicone adhesive designed specifically for applications where a high strength cohesive bond to various substrates is needed.
Click here to Get Your Solution Guide!

Reconstructive / Cosmetic
Chin Implants
Nasal Implants
Facial Reconstruction
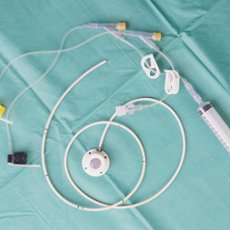
Medical Tubing
Tracheostomy
Endotracheal
Tube Dialysis
Tubing
Shunts
Stents

Audiology
Cochlear Implant

Orthopedic
Joint Implants
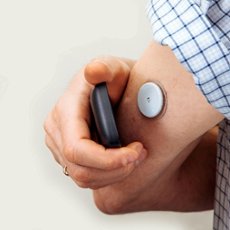
Electronic Devices
Glucose Sensor
Blood Pressure
Heart Rate Sensor
Neurostimulation
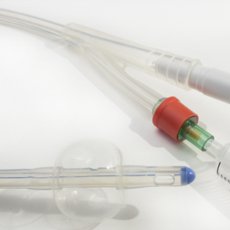
Fluid Delivery / IV Systems
Medical Port
Implantable Infusion Pump
Catheter

Cardiology
Heart Valve
Pacemaker
Ventricular Assist Device

Gastroenterology
Gastric Band
Feeding Tube
Gastric Balloon
Drug Delivery Systems
Intravaginal Ring
Intrauterine Device (IUD)

Experience the Silbione™ Difference
Silbione™ silicones are increasingly used in healthcare and biomedical applications as a result of their biocompatibility, chemical inertness, hypoallergenicity, high performance physical properties and stability across a wide range of environmental conditions.
DOWNLOAD
