COMPANY
INSIGHT

BIOCOMPATIBLE ALD THIN FILM ENCAPSULANTS FOR IMPLANTABLE MEDICAL DEVICES
Atomic Layer Deposition (ALD) is an advanced thin film coating method which is used to fabricate ultrathin, highly uniform and conformal material layers for several applications.
ALD films are dense, defect- and pinhole-free, and their thickness and physico-chemical characteristics can be precisely tailored down to atomic level. Typical film thicknesses range from a few to a few tens/hundreds of nanometers. ALD process can be performed at relatively low temperatures, so it is suitable also for sensitive materials and devices such as plastic- and polymer-based components.
The list of ALD materials ranges from oxides, nitrides, fluorides, carbides, and sulfides to ternary compounds and metals. Several ALD materials are intrinsically biocompatible/bioactive and thus ideal for implantable medical devices.
The number of ALD applications has grown exponentially over the past few years. Originally ramped up to large volume manufacturing in mid 2000’s by microelectronics industry, ALD is nowadays a mature, key enabling technology that realizes our modern, wireless and connected world of mobile communications and more and more efficient computers.
Medical technology is one of the key industries that reaps the benefits of the on-going digitalization and miniaturization of electronics. Instead of heavy surgeries and clumsy, expensive machines, diagnostics and treatments can be performed remotely and with minimally invasive methods.
Remote sensing and therapeutics through self-powered, wirelessly operating microimplants is an emerging technology that is finding a plethora of uses throughout the medical field. Some key examples are neural stimulation and diagnostics, blood glucose, blood pressure, intraocular and intracranial pressure measurements, and even artificial eyesight.
These minuscule devices are typically implanted for extended periods of time, so encapsulation of their sensitive electronics against the corrosive environment inside the human body is crucial. Naturally, also the body has to be protected against possible contaminant leakage, inflammation or rejection reaction caused by the implant.
Traditional encapsulants, mostly used for macroscopic objects such as pacemakers or Cochlear devices, include metals, ceramics, and polymers. Their downside is their thickness and robustness which increases the mass and dimensions of the implant, and decreases its comfort of use. Also, when the implant size diminishes, and the requirements for the implant lifetime increase, novel encapsulation methods are called for. |
Fig.1: The principle of ALD.

ALD offers a truly sophisticated, proven method for reliable, hermetic, protective encapsulation of various implantable devices, from microelectronic sensors to macroscopic items such as orthopaedic and dental implants.
As ALD produces ultra-high quality thin films, that cover uniformly and conformally even complex microscale 3D-structures, the desired effect can be achieved with much thinner material layers compared to traditional methods.
Picosun has worked with medical ALD applications for years, providing production solutions e.g. for coating of dental implants, and for surface treatment of drug particles. For titanium-based dental implants, Picosun’s bioactive ALD TiO2 coating enhances the osteointegration process, whereas biocompatible Al2O3 encapsulation enables design of highly advanced drugs with controllable release time and target.
In addition to TiO2 and Al2O3, also HfO2, SiO2, ZrO2, Nb2O5, Ta2O5, AlN and TiN ALD films manufactured by Picosun have been tested and validated to be non-cytotoxic and safe to human tissues in implant applications (*). This wide variety of materials gives great flexibility in designing novel ALD solutions for a plethora of healthcare uses, when the materials can be used either as such, or combined into nanolaminates or doped films with unique, application-wise tailorable properties.
Picosun’s newest offering to the medical industries is an ALD-based nanolaminate encapsulant, that can potentially ensure microimplant lifetime of over 10 years in human body environment (**). The nanolaminate deposition process is readily scalable to high throughput, cost-efficient industrial production of thousands of implants per run in PICOSUN® P-300B or P-1000 ultra-large batch ALD reactors.
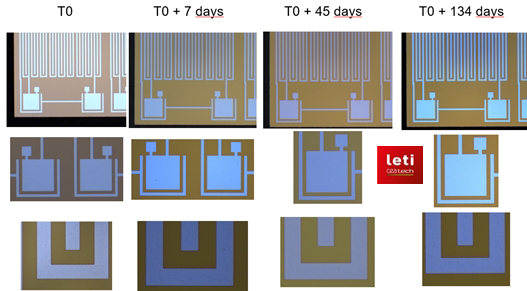
Fig.2: Examples of Picosun’s ALD materials’ performance in implant encapsulation (i): Microimplant electronics protected by Picosun’s ALD HfO2. No changes after soaking in 87 oC PBS for over 3 months which correlates to over 10 years in human body. T0 = starting point of the test. Reference: InForMedproject, image source CEA-Leti.
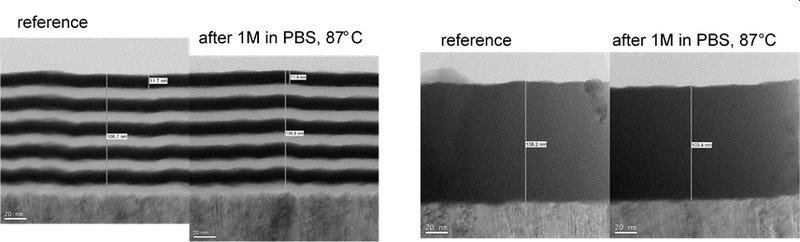
Fig.3: Examples of Picosun’s ALD materials’ performance in implant encapsulation (ii): TEM micrographs of Picosun’s ALD nanolaminate and oxide encapsulants after one month soaking tests in 87 oC PBS. No corrosion observed.
(*) FICAM – The Faculty of Medicine and Health Technology, University of Tampere, Finland: Cytotoxicity tests with cell culture medium according to the ISO 10993-5 standard, and 3 weeks soaking tests in PBS (phosphate-buffered saline) at 87 oC.
(**) J. Jeong et.al: Conformal Hermetic Sealing of Wireless Microelectronic Implantable Chiplets by Multilayered Atomic Layer Deposition (ALD), Adv. Funct. Mater. 2018, 1806440. DOI: 10.1002/adfm.201806440.
About Picosun Group:
Picosun Group is the leading provider of AGILE ALD® (Atomic Layer Deposition) thin film coating technology for global industries.
As the inventors and pioneers of the technology for over four decades, our aim is to take ALD to always new frontiers, by working in close collaboration with our customers. Together, we create the production-proven, cutting-edge ALD solutions to revolutionize future industries.
PICOSUN® product portfolio ranges from fully automated, large scale industrial batch ALD systems to smaller scale research and pre-pilot production tools. Our turn-key ALD solutions combine agile, compact and innovative equipment design, the leading process quality, and the most comprehensive strategic partnership throughout your ALD journey.
Picosun is based in Finland, with subsidiaries in Germany, North America, Singapore, Taiwan, China and Japan, offices in India and France, and a world-wide sales and support network.
