Company Insight
Sponsored by Alleima
Hope for Parkinson’s patients: The brain implants changing lives
Brain implants are making a life-changing difference for people with Parkinson’s. While drugs may have a limited effect, these devices are ensuring that diagnosis of the condition does not mean a debilitating decline and support patients in getting back to a normal way of life.
Advanced devices are now giving hope to greater numbers of patients living with Parkinson’s disease. Millions of people worldwide are affected by the chronic and progressive neurological disorder.
The disease predominantly affects the motor system, leading to symptoms such as tremors, rigidity, and bradykinesia, and originates from the degeneration of neurons in the substantia nigra, a part of the brain that is crucial for regulating movement.
Traditional treatments have largely revolved around medications and physical therapy, but recent advancements in neurotechnology, specifically brain implants, are starting to significantly alter the treatment landscape.
According to GlobalData’s Parkinson’s disease global drug forecast and market analysis [1], treatment rates vary depending on the severity of the disease and the region. Early-stage patients receive drug therapy in 74%-88% of cases, and this rises to 82%-95% in advanced-stage patients. Approximately 5%-10% of patients are referred for a deep brain stimulation (DBS) procedure, representing more advanced and severe cases.
Parkinson’s prevention and disease-modifying drugs
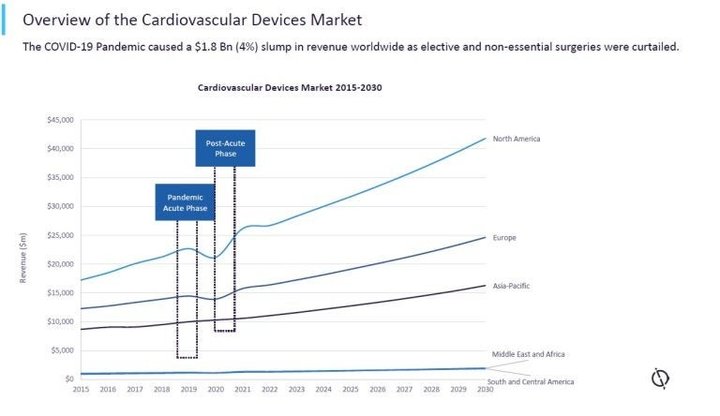
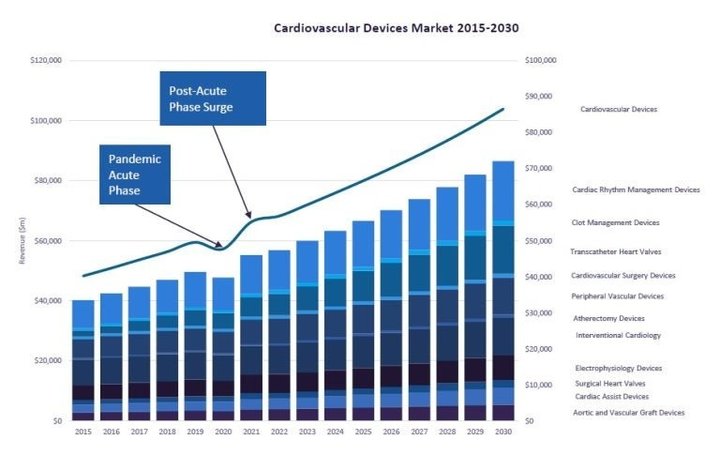
The Covid-19 pandemic caused a global shock to the medical services market. In some areas, this entailed a sharp surge in demand, leading to lowered inventories and a scramble to fulfil orders. In many areas, disruption was in the form of a sudden drop in demand, with elective and non-essential surgeries curtailed. According to GlobalData analysis, some of the market segments that saw the biggest fall were interventional cardiology, cardiac rhythm management, surgical heart valves, clot management devices, and atherectomy devices. As the pandemic progressed, some restrictions were relaxed to enable hospital procedures to resume.
Parkinson’s disease prevention requires a combination of early diagnosis and the development of disease-modifying drugs that can halt the progression. However, a key challenge in the development of these drugs for Parkinson’s is the lack of valid biomarkers that can measure changes in disease progression in the early stages of the disorder.
The current clinical diagnosis methods for Parkinson’s have significant shortcomings with limited accuracy. As a result, the prodromal stages are challenging to capture. Furthermore, early symptoms are frequently missed or misdiagnosed.
“The development of predictive biomarkers of Parkinson’s progression would have multiple implications in developing disease-modified therapies for Parkinson’s disease,” comments Christie Wong, GlobalData pharma analyst.
These implications include improvements in diagnosis, allowing for earlier intervention, improved disease staging and stratification of patient populations, better clinical trial design, and an ability to predict patient prognosis. In addition to clinical benefits, biomarkers would likely enhance scientific advancements in the understanding of Parkinson’s pathophysiology.” [2]
Deep brain stimulation: Can we reverse symptoms in advanced Parkinson’s disease?
DBS involves surgically implanting a neurostimulator that sends electrical impulses to targeted areas in the brain. These impulses regulate abnormal impulses, or fill in where impulses are lacking, effectively helping to smooth out and improve motor functions.
An implant is typically placed in one of three brain regions depending on the patient’s symptoms – either the subthalamic nucleus, the globus pallidus internus or the thalamic region. It is connected to a battery-operated medical device implanted in the chest that controls the amount of stimulation delivered based on the patient’s needs.
In its global drug forecast report, GlobalData asked several key opinion leaders why rates for DBS referrals are lower than 10%. They reported that this solution is almost exclusively reserved for advanced cases. Drawbacks include requiring invasive surgery, lack of availability of qualified neurosurgical teams, and high costs. However, as ongoing advancements in technology are making the devices smaller, and 5G-enabled surgical robots make remote surgery possible, DBS could become a viable option for more patients.
Changing lives with deep brain stimulation devices
Parkinson’s is often assumed to only affect the elderly. Yet Andrew Johnson, a successful lawyer from New Zealand, had his life changed when he was diagnosed at only 35. In the years that followed, the cognitive challenges were becoming insurmountable, forcing Johnson to leave his job and severely impacting his day-to-day life.
Two years after his diagnosis, Johnson’s neurologist suggested the possibility of DBS surgery. Typically, patients are not considered for DBS until at least ten years post-diagnosis. However, research indicates that earlier intervention may prolong the longevity and efficacy of treatment in early-onset cases.
“DBS surgery was life changing. It gave me control over my body again,” stated Johnson. “The way my motor symptoms were going, I think I would have had to be in full-time care if it wasn’t for the DBS. I try to take advantage of the time I have been given and live life like I really mean it.”
In some cases, DBS may not be an option – such as for one patient, who already had an implant that would have been too difficult to remove. Instead, Marc Gauthier, 63, became the first person to try a new implant that stimulates nerves in the spine, which has helped him regain the ability to walk. Gauthier had previously been housebound but is now able to walk for miles.
Innovations in DBS devices
The conductive wire used in DBS devices has several stringent requirements, something that becomes increasingly complicated as devices get smaller.
Alleima manufactures ultra-fine high-quality wires for use in DBS and spinal cord stimulation devices that are both highly durable and conductive. The company has an extensive range of stainless steel, precious metals, and CoCr alloys, as well as coatings and surface treatments. Alleima works closely with customers to configure medical wire components that are perfectly calibrated for DBS and spinal cord stimulation (SCS) applications.
Commonly suggested wire materials for DBS and SCS applications include platinum-iridium alloys, which offer excellent biocompatibility along with radiopacity, moderate strength and good formability. To maximise fatigue strength for implantable devices, these platinum-iridium alloys are delivered with the highest surface finish – Medical class.
To find out more about Alleima’s wires are advancing healthcare, from DBS devices to continuous glucose monitors, download the whitepaper below or read more on their website www.alleima.com/medicalwire.
Contact information
Alleima Advance Materials
1 Commerce Blvd.,
Palm Coast, FL, 32164,
United States
Tel: +1 386 445-2000
Fax: +1 386 447-5113
Email: ms.spc@alleima.com
Alleima Tucson
2424 East Aragon Rd
Tucson, AZ 85756
United States
Tel: +1 520 495 5927
Alleima Zug
Oberallmendstrasse 20a
CH-6300 Zug
Tel: +41 41 761 63 55
Email:sales@alleima.com
Alleima St Imier
Rue de Beau Site 8
CH-2610 Saint-Imier
Tel: +41 32 942 39 20
Email: info@alleima.com