COMPANY
INSIGHT
Regulatory Globe – Your Regulatory Partner for Medical Devices
Regulatory Globe provide regulatory affairs knowledge to medical device companies on a digital way.
Did you know? According to EU MDR / IVDR manufacturers shall have available within their organization at least one person responsible for regulatory compliance and micro and small enterprises need at least such person externally with permanent access.
Who are we?
Welcome to the digital age. Regulatory Globe provides digital Regulatory Affairs products and services to help companies to be compliant with all necessary regulations, guidance’s and standards and to consolidate and establish regulatory knowledge in your company.
Regulatory situation in EU:
As you are aware, the European medical device industry will undergo significant change as a result of the new medical device regulation which is currently finalized since May 26th, 2017. As the name suggests, it is a regulation and no longer a directive and all medical device companies have to adhere to this new regulation. Companies not following the new rules will no longer be allowed to sell their medical products in the European Union. This legislation change have a big influence on European businesses as well none EU-businesses which have their products on the European market. All these new regulations and requirements takes a lot of effort and knowledge to bring all together to be prepared for these huge regulatory waves.
Our Experiences:
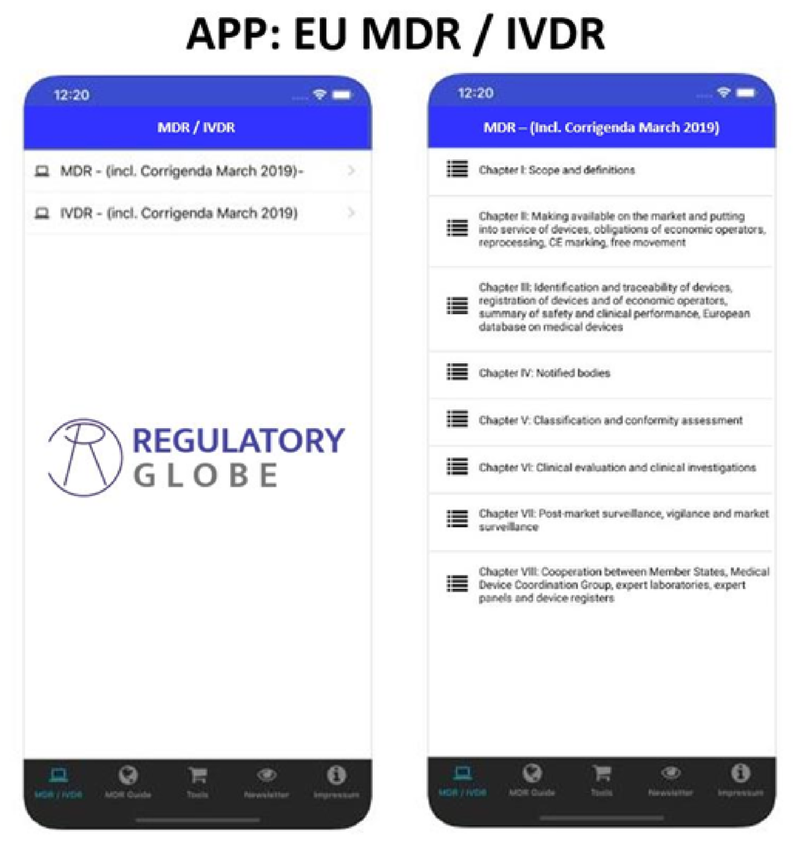
Regulatory Globe is specialized in Regulatory Affairs knowledge in the Medical Device field and is the leader in providing EU MDR and IVDR Gap-Assessment Tools and has a strong relationship with several known partners across the globe, to be able to provide regulatory affairs support from A to Z.
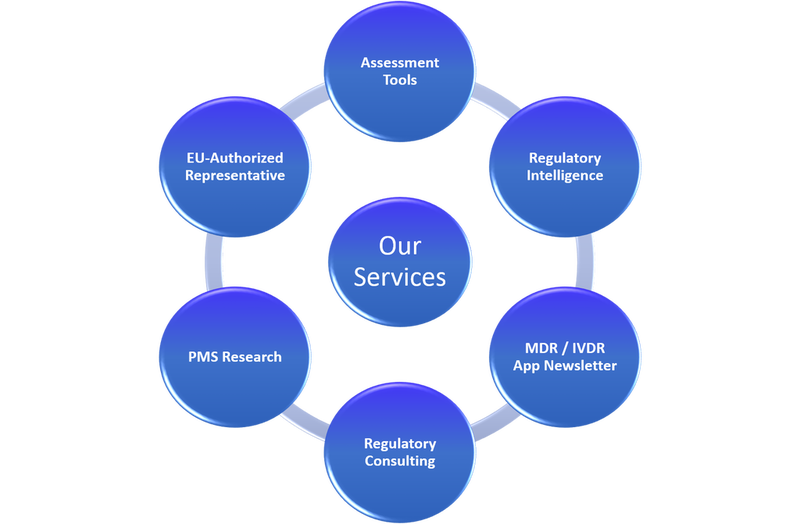
Our Services:
In the table below are listed our services. If you need more information, please don’t hesitate to contact us.
Service:
Details:
Link:
Assessment Tools: |
- EU MDR Gap-Assessment Tool
- EU IVDR Gap-Assessment Tool
- MDSAP Checklist
- ISO 13485:2016 Checklist
- PMS
- And more…
Link to overview:
|
- Monthly Regulatory Affairs updates from USA, Canada, Brazil, Japan, Australia, EU, UK and Switzerland.
Link to overview:
Go to Regulatory Intelligence Paper
|
|
Contact: info@regulatoryglobe.com
OUR VISION
Health is one of the basic needs of humans and should always come first. People who cannot cover these basic needs, for whatever reason, are usually dependent on outside help and that is precisely the goal of the medical technology industry. To develop medical products that improve quality of life and extend life expectancy.
In order to serve this basic need optimally, laws and regulations are inevitable. Such regulations ensure safety and quality of products as well as raise the state of the art and thus promote progress steadily. This is the reason why it is so important to take requirements as an opportunity for improvements and not as a burden.
This is where the task of Regulatory Globe GmbH begins. Together with our partners, we specialize in monitoring laws and regulations and provide tools that help our clients improve their products processes. This will eventually benefit the people in need of these medical devices.

Michael Galliker
Founder and CEO