
Visit our booth at
- MedtecLIVE, Nuremberg, 31.3 - 2.4.2020, Booth 9 - 217
- T4M, Stuttgart, 5+6.5.2020, Booth in Start - up World
Why welding
plastics by laser?
Turnkey S

Turnkey M

Modula

Welding plastics in medical technology
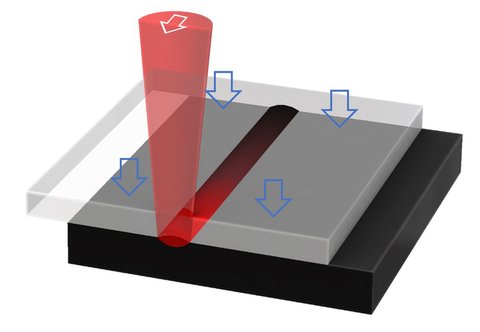
For medical devices laser welding of plastics
primarily scores with its precise and clean welds.
The necessary energy to melt the plastics is only very local without any heat impact on nearby areas as e.g. heat-sensitive reagents.
Compared to ultra-sonic welding no particles are generated and no vibrations created, which may damage sensitive electronics.

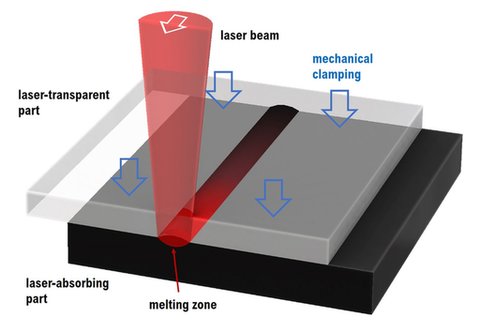
Advantages
• precisely localized
• shallow melting zone
• little energy required
• no particles
• no vibrations
• no emissions
• no solvents
more information …
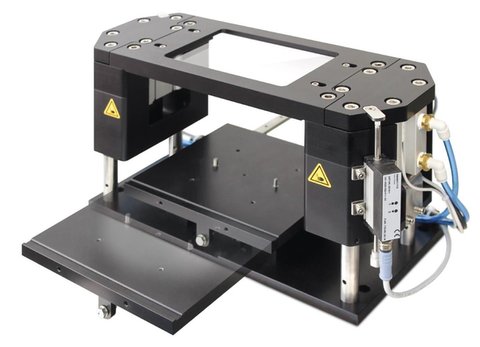
Turnkey S
The "Turnkey S" is a small ready-for-use machine for laser welding of plastics. With its flexibility by modular design and its compact size, it is unique on the market.
It is based on a modular building framework, so that it can be offered in
various configuration depending on the parts to weld and the customer requirements.
It can be equipped with different laser powers and motions systems.
With servo-axes for contour process an area of 100x150 mm can be covered and with an optional z-axis also different height levels reached.
With a scanner optics for quasi-simultaneous welding process an area of up to 100x100 mm is possible.

more information …
Turnkey M
The "Turnkey M" is a ready-for-use machine for larger parts up to half a meter in size. It is designed as a workstation that can be operated in standing or sitting.
Similar as Turnkey S the Turnkey M is based on a modular framework and can be configured in various versions.
Besides different laser powers, optics, and motion system, it can be equipped with a simple drawer for the handling of the workpieces to weld or with a two position rotary table.


more information …
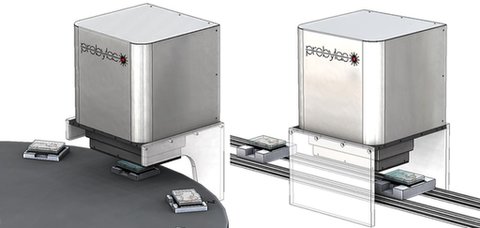
Modula
The “Modula" components are for special machine building if the Turnkey systems do not fit the specific requirements.
The laser unit with process control inside and the optics are always required. Additionally, clamping units and motion systems are supplied to provide complete process sub-units so that special machine builder needs only to care about the handling of the work-pieces in and out and the machine safety.




more information …
