COMPANY INSIGHT
Sponsored by Duke Empirical
DUKE Empirical
is a leading catheter and medical tubing contract manufacturer providing design, development, and validated production within an FDA registered and EN 13485 certified facility.
F
ounded in 2000, DUKE has since established the shortest lead times in the industry! Our vertically integrated process capabilities speed the development of innovative catheter and medical tubing products from concept to commercialization and shorten our customer’s supply chain throughout the entire product lifecycle. DUKE Extrusion is the trusted industry brand representing the medical tubing and extrusion business unit within the company DUKE Empirical, Inc.
PRODUCT AND SERVICE OFFERINGS
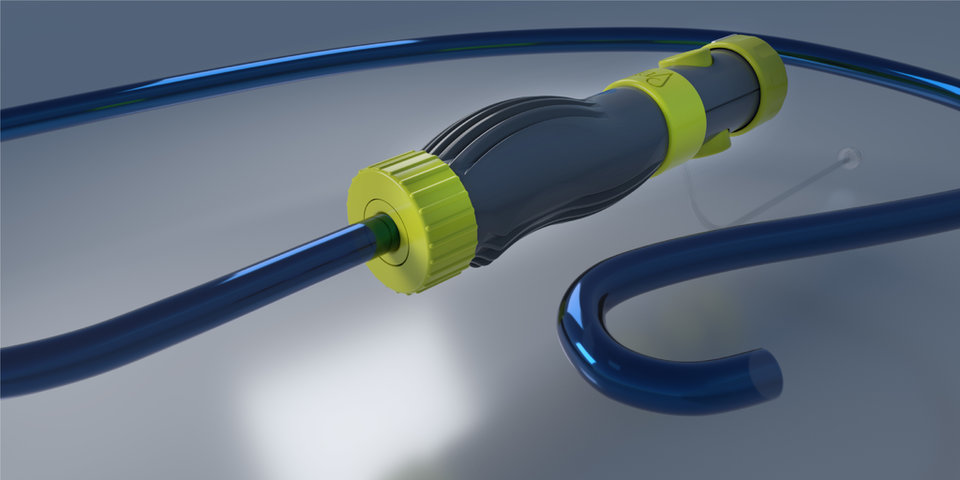
- VERGO® Steerable Access Sheaths - Off-the-Shelf availability with a range of product sizes from 18Fr through 32Fr. Custom steerable catheter systems are also available from existing designs.
- Precision custom medical extrusion – multi-lumen, coextrusion, high temp and fluoropolymer materials
- Over 3,000 standard tubing products including braid reinforced catheter shafts and polymer catheter tubing- available for immediate purchase through the DUKE Extrusion online store www.dukeextrusion.com/store
- In-house custom catheter and component design, development, and manufacturing
- Innovative proprietary low friction materials – PebaSlix®, PolySlix®, NyloSlix®, and FluoroSlix®
- Design verification testing and process validation services
- Finished Goods- packaged, labeled and sterile
MARKETS SERVED
Structural Heart Therapies – transcatheter heart valve applications
Interventional Cardiology
Interventional Neurovascular Devices
Vascular Access Solutions
ENT
Gastro-Intestinal Interventional Devices
Drug Delivery
Oncology
Interventional Pulmonology
And others
CAPABILITIES
- Braiding & Coiling – wire and filament
Hydrophilic Coating – application of Biocoat’s Hydak® Coating System
Laser Welding and Laser Cut Hypotubing
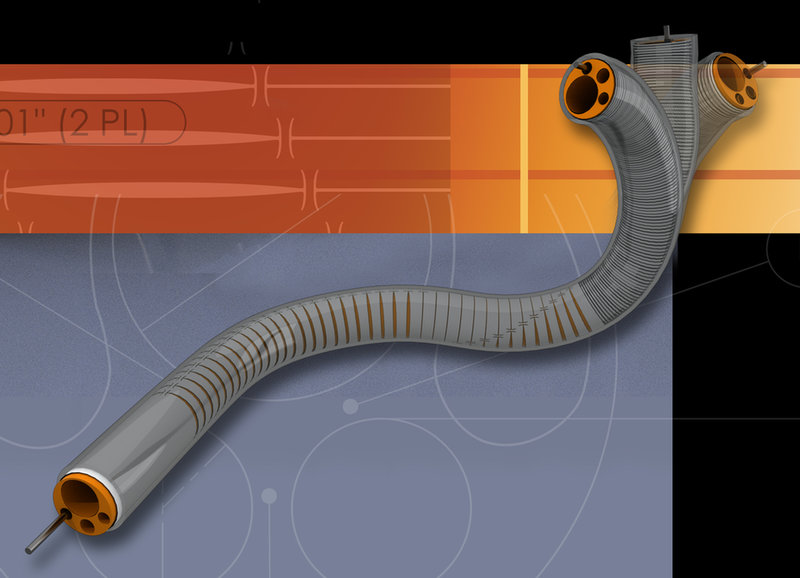
RAPID PRODUCT DEVELOPMENT
DUKE maintains a dedicated team of R&D engineers specifically devoted to the development of novel and early stage medical device designs. With a broad range of medical grade materials stocked and ready for prototyping, as well as custom extrusion and tubing expertise, our engineering team conceives, develops, and builds new designs in days!
DUKE utilizes multiple rapid prototyping machines and methods such as Digital Light Projection, Fused Deposition Modeling, and CNC machining. These in-house technologies allow for the rapid development of catheter components, functional ergonomic handles, and user interfaces for catheter devices. Direct injection molding using printed or CNC machined molds is provided for quick turn low volume prototypes made of medical grade polymers suitable for design evaluation with bench testing and in-vivo feasibility trials.
CONTRACT MANUFACTURING
DUKE Empirical’s commitment to product quality and on-time delivery is realized in our manufacturing services. Production takes place within ISO Class VIII Controlled Environment Rooms (CER) which are maintained according to an externally certified Quality Management System. Utilized by a majority of world leading medical device original equipment manufacturers, DUKE is a trusted partner producing innovative, cutting edge catheter and tubing products in high volumes and which are used daily in markets throughout the world.
Continuous quality improvement, lean manufacturing principles, and validated processes using the latest in automation technology combine to ensure product performance, consistency, and cost efficiency. Our mission is to deliver valuable customer solutions to the medical device industry through the design, development, and manufacture of innovative products. As such, our customer’s requirements are integrated into every process and product that DUKE manufactures.
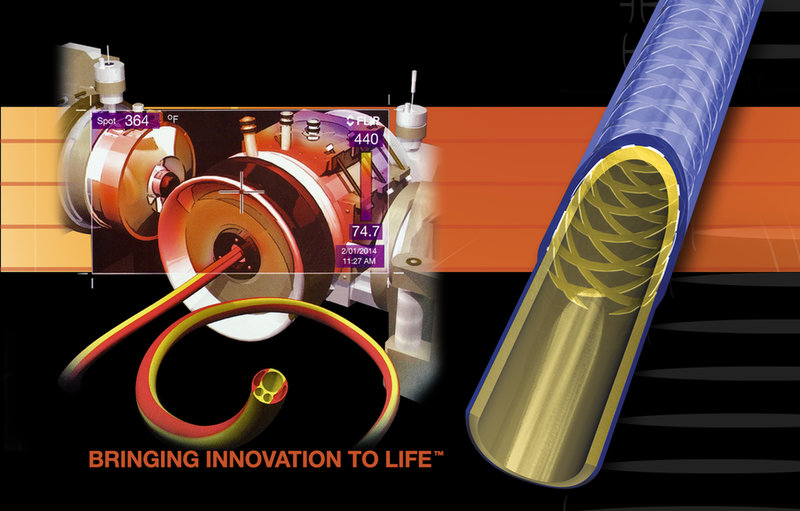
BRINGING INNOVATION TO LIFE!
With our focused expertise in catheter design, materials engineering, and advanced proprietary processes, DUKE has achieved unparalleled results for our customers. Our innovative products allow for the design of tomorrow’s medical devices that push the limits of lubricity, flexibility, torque response, and deliverability all within the smallest possible profile. Leverage our experience and expertise; contact us today and learn how our team of dedicated medical device professionals can accelerate your new product introduction! We look forward to the opportunity to exceed your requirements and expectations!
Contact information
Contact us today to review your project and see how our expertise in catheter devices and medical tubing can improve your product; or Request a Direct Pricing Quote or Material Sample Request today!
Visit www.dukeempirical.com , or www.dukeextrusion.com , or contact us by email at customerservice@dukeempirical.com , or by phone (831)-420-1104.