COMPANY
INSIGHT
The questions were answered by Konrad Betzler, Chief Pharma Officer of Haselmeier GmbH & Co. KG.
Answered by Konrad Betzler, Chief Pharma Officer of Haselmeier GmbH & Co. KG.
Accumold began more than 30 years ago with an idea that there had to be a more efficient way to produce small and micro-sized plastic molded parts. The services that were available at the time proved to be insufficient to handle this new demand for high-precision, micro molded components. This challenge in 1985 sprouted Accumold in to action and the first truly dedicated micro molding machine was developed.
In the mid-1980s the micro-electronics boom we know today was still in its infancy. Accumold was ahead of the curve but could see the wave of demand that devices were getting smaller and smaller by the generation. This head start gave the company a huge advantage in perfecting the art and science of micro injection molding. Along the way the company grew and expanded. From their rented garage to the state-of-the-art facility they call home today Accumold has become a world-class molder.
As was in the beginning, Accumold’s single focus is still dedicated to micro molding. The company’s innovations over the years have continued to push the limits of micro plastics. Their expertise in producing complex, tight tolerance and micro-sized moldings is second to none and is sought out by design engineers and development teams worldwide.
Mr. Betzler, what significance does the time factor have for clinical trials
Answered by Konrad Betzler, Chief Pharma Officer of Haselmeier GmbH & Co. KG.
Whoever is first on the market with a new therapy method determines the price. Every successor thereafter must make a lot of effort to gain market shares of the initial supplier. The speed is important even for generic drugs / Biosimilars: In the US, for example, the first generic drug has exclusive market access for six months. Therefore, every company will make the best efforts to ensure a quick start and a smooth conduct of the clinical trials.
A further important aspect in clinical trial studies is the actual quantity of the new medication available. At the beginning of the clinical research only small quantities of the active ingredients are available – the trial drug is therefore expensive and scarce. It is not always easy to find patients, and studies have to be carried out in many countries. Therefore, it is desirable that as far as possible the trial drug can be used globally. That poses enormous challenges for the labelling of drugs, and also for the approval status of medical products and resources which are required for the study.
This platform can be flexibly configured to suit the desired dose values, from the first clinical study to series production, thereby significantly reducing the “time to market” and “CAPEX”.

What role do the pens play as an injection aid
Answered by Konrad Betzler, Chief Pharma Officer of Haselmeier GmbH & Co. KG.
If the drug has to be injected subcutaneously, injection pens offer a great opportunity to control the time factor. You see, generally vials are used during the clinical phase. It is the fastest method of treating patients with drugs to be injected below the skin. The doctor draws up the medication into disposable syringes and administers it to the patient. The production is relatively simple and the dose can practically be adapted as desired. Vials are however rather inconvenient to use and harbour safety risks. Some of the risks include shelf-life after opening, particles as contamination when piercing the skin, injury to the staff due to the cannula. An alternative is the pre-filled syringe, which does not however permit any dose adjustment and thus would mean a substantial additional demand of the (expensive) trial drug. What is common to both forms of dosage is that they are rather unsuitable for use by medical laymen. A usage once or several times a day therefore requires substantial nursing care and virtually hospital treatment.
As against that, self-application by the patient, such as with injection pens, has several advantages: The patient is flexible in terms of place and time and the care required, and thus the therapy costs are reduced greatly. It should be possible to show this cost efficiency today itself within the framework of the clinical studies. A change from syringe to injection pen just shortly before the market-launch creates further uncertainties and thus extends the time for approval. If pens are already established in the market as a pharmaceutical form for certain indications (e.g. diabetes I and II), there is hardly the option of reverting to vials or pre-filled syringes during the clinical testing – neither doctors nor patients would accept that. When the medical prerequisites for the application of a medication as pen are given, the pen should therefore be used as pharmaceutical form as early as possible in the clinical studies.
The especially flexible D-Flex disposable pen has been awarded the GOOD DESIGN™ award 2016.
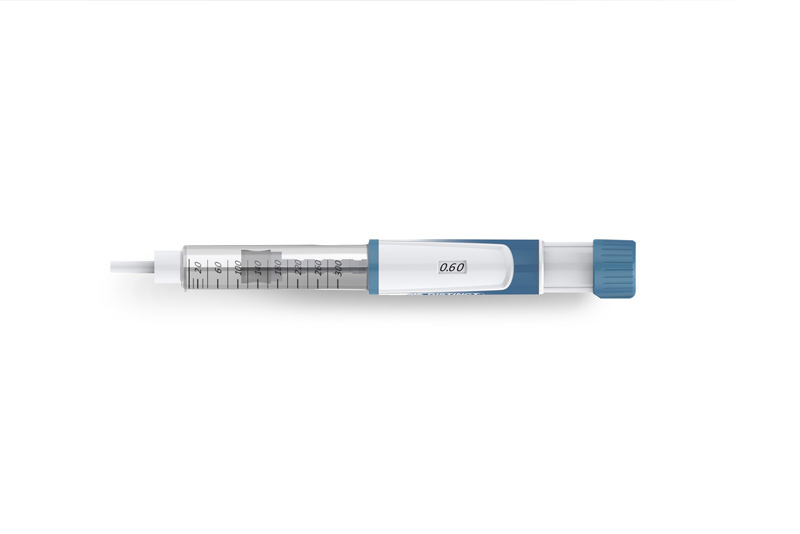
What are the demands that a clinical supply manager makes on a pen for clinical studies
Answered by Konrad Betzler, Chief Pharma Officer of Haselmeier GmbH & Co. KG.
- A pen, which requires no or a minimum effort during the trial and certification of the new medication and is permitted in the countries in which the studies are being conducted.
- A pen, that is simple and safe to use, and still permits a pre-defined flexibility in the adjustment of the dose.
- A pen, that permits a therapy appropriate labelling, during printing of dose adjustment as also the option of multi-lingual labels.
- A pen, that can be used continuously or with minimum adjustments for all phases of the clinical study up to the approved drug.
- A pen, that can be delivered ready-to-use for the test persons, without having to establish a manufacturing competence in house.
The D-Flex dose configuration tool provides information as to whether the desired dose values can be selected during the assembly of the pen.

What are the demands that a clinical supply manager makes on a pen for clinical studies?
- A pen, which requires no or a minimum effort during the trial and certification of the new medication and is permitted in the countries in which the studies are being conducted.
- A pen, that is simple and safe to use, and still permits a pre-defined flexibility in the
- adjustment of the dose.
- A pen, that permits a therapy appropriate labelling, during printing of dose adjustment as also the option of multi-lingual labels.
- A pen, that can be used continuously or with minimum adjustments for all phases of the clinical study up to the approved drug.
- A pen, that can be delivered ready-to-use for the test persons, without having to establish a manufacturing competence in house.
The D-Flex dose configuration tool provides information as to whether the desired dose values can be selected during the assembly of the pen.

How does Haselmeier meet these requirements
Answered by Konrad Betzler, Chief Pharma Officer of Haselmeier GmbH & Co. KG.
Haselmeier has developed a new, innovative product, the D-Flex, which is highly suitable for clinical trials. This is a disposable pen for use with 3ml cartridges. It bridges the gap between fixed-dose pens, which only allow only a single fixed dose, and variable-dose pens, where the dose can be relatively freely adjusted by the patient. The D-Flex can therefore be configured for several fixed doses. These dose values can essentially be freely selected when designing the pen. This is especially of interest for dose escalation studies. The pen system does not allow any intermediate steps between the set doses. This significantly reduces the risk of any wrong dose and enhances the safety for the patient.
This makes the D-Flex the ideal, flexible platform for adapting to set doses in accordance with the therapy. In the process it has been so well developed and validated that only minimal molecule-specific and customer specific adjustments are necessary, which can largely be integrated into the clinical supply chain process seamlessly up to the serial production after the market authorisation. And you can have it finally assembled at Haselmeier up to the drug-device combination product.
Subcribe to Haselmeier:
View Haselmeier’s products:

The especially flexible D-Flex disposable pen has been awarded the GOOD DESIGN™ award 2016.
How does Haselmeier meet these requirements?
Haselmeier has developed a new, innovative product, the D-Flex, which is highly suitable for clinical trials. This is a disposable pen for use with 3ml cartridges. It bridges the gap between fixed-dose pens, which only allow only a single fixed dose, and variable-dose pens, where the dose can be relatively freely adjusted by the patient. The D-Flex can therefore be configured for several fixed doses. These dose values can essentially be freely selected when designing the pen. This is especially of interest for dose escalation studies. The pen system does not allow any intermediate steps between the set doses. This significantly reduces the risk of any wrong dose and enhances the safety for the patient.
This makes the D-Flex the ideal, flexible platform for adapting to set doses in accordance with the therapy. In the process it has been so well developed and validated that only minimal molecule-specific and customer specific adjustments are necessary, which can largely be integrated into the clinical supply chain process seamlessly up to the serial production after the market authorisation. And you can have it finally assembled at Haselmeier up to the drug-device combination product.
Subcribe to Haselmeier: www.haselmeier.com/see-the-one
View Haselmeier’s products: www.haselmeier.com/produkte/d-flex-2