COMPANY
INSIGHT
Selecting the Best Material for the Next Generation of In Vitro Diagnostics
“In vitro diagnostics” (IVDs) is a term for a broad industry encompassing benchtop or larger bioanalytical instrumentation to point-of-care devices. Microfluidics, or lab-on-a-chip technology, is a powerful tool that sits at the intersection of biotechnology, automation, and functional integration. By using photolithography and other microfabrication techniques to make fluid flow cells, microfluidic devices can shuttle picoliter or smaller volumes into functional regions, enabling everything from synthesis based sequencing (SBS) to organs-on-a-chip. There are challenges with this approach. The viability and functionality of biological material places new burdens on device materials and fabrication requirements that few single foundries can meet. Scalable selective surface modification is becoming key to successful IVD device production.
IMT Masken und Teilungen AG is a foundry for glass components offering exactly that service.
OVERCOMING OBSTACLES FOR IVD DEVICES
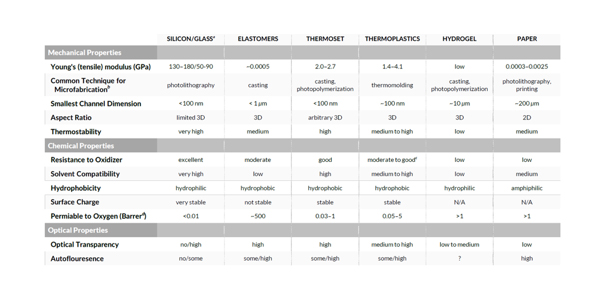
Choosing the best material for a microfluidic flow cell for IVD devices can be the first step in guaranteeing its performance within budget and schedule. Silicon is most useful for high aspect ratios that can be etched using anisotropic chemical etch or reactive ion etch. Thanks to advanced laser microfabrication, high aspect ratio features in glass are more feasible. Glass and silicon are easy to modify with additive methods such as chemical vapor deposition in order to add other materials, metals, and dielectric coatings for additional functionality such as electrodes or waveguides.
Silicon oxide layers created on silicon introduce regions that have some glass-like properties. The ability to create three dimensional structures, for example, to create a scaffold for cells, makes silicon a strong choice for some organ-on-a-chip applications. However, soft lithography materials such as PDMS, and 3D printed materials such as hydrogels, biopolymers, and cells, are attractive because of their biocompatibility and porosity to metabolic gases.
Hybrid materials – glass on silicon, silicon on glass, and plastic on glass, can be constructed to enhance surface properties that are more favorable to the bioassay. Post-fabrication modification using silanization chemistry to create hydrophilic, hydrophobic, or chemically reactive surfaces is best understood for glass.
Polymers have their sweet spot where the cost for millions of disposables is hard to beat using glass or silicon. The cyclic polyolefins can give a sufficient optical transparency and auto-fluorescence for many applications.
caption
Applications such as next generation sequencing (NGS), cell selection and organ-on-a-chip technologies all benefit from the ability to pattern nanofeatures in glass microchannels. NGS transduction approaches may have challenging detection requirements such as measuring single oligonucleotide fluorescence with SBS or electron tunneling through bases as ssDNA traverses nanopores. Additionally, cell encapsulation, droplet digital PCR, sample-to-answer qPCR and digital PCR microfluidic cartridges, encompassing cell enrichment, isolation, lysis, biomolecular sample prep, and PCR amplification / quantitation require partial functionalization and integration of electronic components.

caption
Cost effective glass components for life science
The ease of glass surface modification with a variety of materials, coupled to its mechanical, thermal and optical properties, makes glass a material equal to the biological complexity and engineering complexity of many IVD applications. By employing thermally and UV-A-cured adhesives, it is possible to perform room-temperature bonding processes on glass, allowing for bio-molecule encapsulation prior to bonding. The automation of UV-adhesive bond equipment simplifies and reduces costs for manufacture. Finally, glass can be sterilized by a variety of methods, a critical part of IVD manufacturing.
Remember the interface between the microfluidic device, which is a component, and its packaging. The interface between device and its package is decided on a company-by-company and product-by-product basis. Here pick-and-place technologies and other integration processes can facilitate scalable production of complete flow cells, including packaging and sterilization.

caption
Economies of Scale
Material selection determines the production scale processing methods that affect design, cost, and quality. Glass is the best material for silane chemistry, room temperature bonding techniques, and coating methods for patterning devices from the prototyping to the manufacturing scale. The ideal IVD foundry offers a one-stop shop for bio-friendly negative and additive manufacturing steps, the adhesion layer requirements for the underlying material functionalization, and the final removal of each component from the wafer for individual packaging. That ideal foundry also provides a multifunctional substrate with patterned bio-functionalization, removing complex manufacturing steps for the IVD device developer, who can then add their own proprietary functionalization chemistry.

caption
Applied Harmonics:
The company’s production environment – including 1300m2 clean rooms and a fully automated process line for 200mm glass wafers - is well suited for mass production of lab-on-a-chip glass components and flow cells containing micro-channels, metallic and dielectric microstructures in combination with waveguides and gratings.